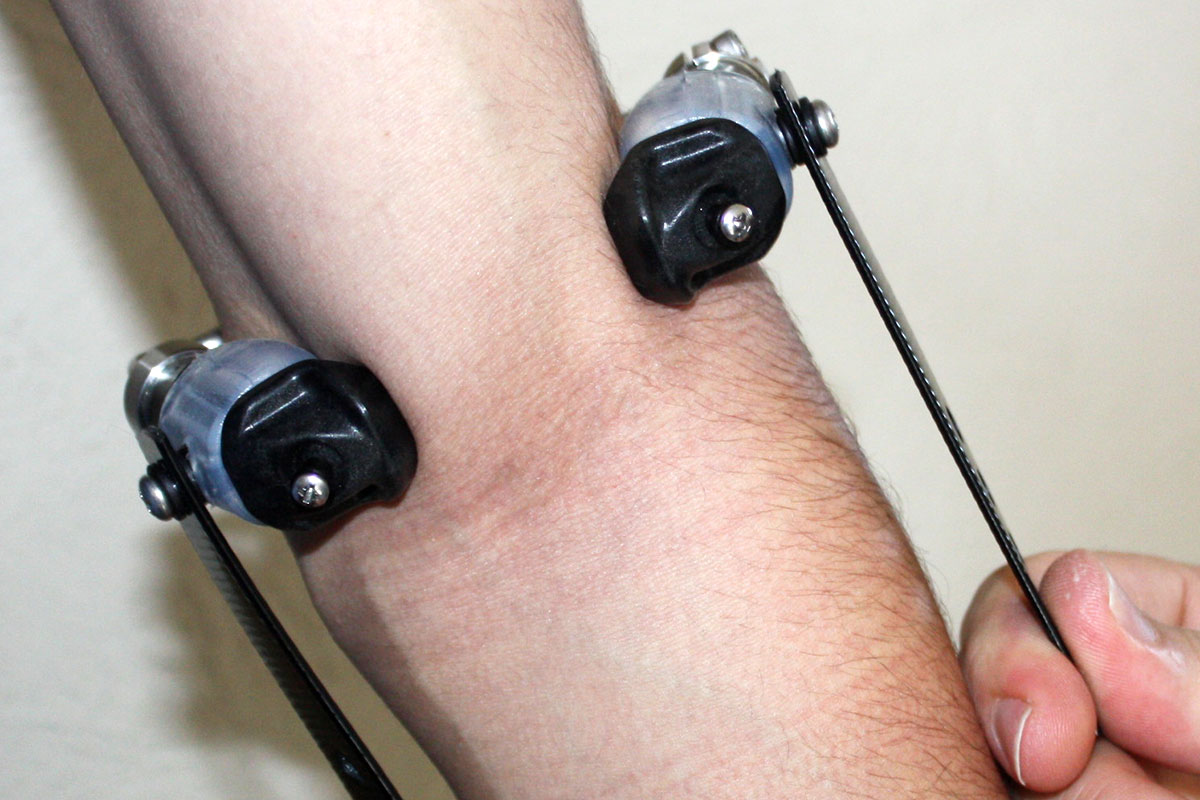
Humeral Suspension Cuff Arm Prosthetic
ToughWare’s patented (US Pat. 8,414,658) Humeral Suspension Cuff (HSC™) provides exceptional suspension while preserving elbow full ROM. Like a three-jaw chuck, the HSC engages the distal humerus, eliminating the need for suspension straps or harnessing that can cause secondary problems with the neck and shoulder muscles.
Description
ToughWare’s patented (US Pat. 8,414,658) Humeral Suspension Cuff (HSC™) provides exceptional suspension while preserving elbow full ROM. Like a three-jaw chuck, the HSC engages the distal humerus, eliminating the need for suspension straps or harnessing that can cause secondary problems with the neck and shoulder muscles. Once correctly adjusted, the HSC is easily donned and doffed (no tools required) and provides comfortable suspension. Originally developed for the ITAL, clinicians worldwide now use the HSC to add adjustable supracondylar suspension to sockets they craft for their patients including both body-powered and externally powered prostheses.
- Maintains suspension at full elbow extension, unlike Muenster and Northwestern sockets.
- Permits unimpeded, full ROM, air circulation, and sweat drainage for increased skin health.
- Open-frame design keeps elbow uncovered, preserving tactile sensation.
- On-patient adjustments permit iterative tuning and verbal feedback for optimal comfort.
- No repeated casting with check sockets to address residuum volume changes.
- Suitable for use with both body-powered and myoelectric prostheses.
- Eliminates socket rectification guesswork to achieve excellent supracondylar suspension.
HSC units normally come with a flat PLUG back; if a control cable anchor point is required, SWIVEL attachment and conventional CROSSBAR attachment options are available as add-on kits.

ToughWare’s HSC

Shown in place on arm

Making an adjustment

HSC with PLUG back

with SWIVEL

with CROSSBAR
HSC with PLUG back (bottom-left), with SWIVEL (bottom-center), and with CROSSBAR (bottom-right).
Part Number & Ordering Information


In the event a user experiences extreme skin irritation, impaired circulation, pain, or any condition they or a clinician deems excessive or unsafe, immediately discontinue use of the HSC and remove the device from the user’s residual limb until the cause is identified and the problem is corrected.
For the user’s safety, they must be able to put on and take off the HSC by themselves without the use of tools, having to loosen parts of the HSC, and/or without having to bend or distort the device. If they are unable to do so, the HSC is unsuitable for this individual and must not be prescribed for their use or be used by them.
In the event a user experiences extreme skin irritation, impaired circulation, pain, or any condition they or a clinician deems excessive or unsafe, immediately discontinue use of the HSC and remove the device from the user’s residual limb until the cause is identified and the problem is corrected.
HSC Parts

HSC Strap Kit

HSC Swivel Control Cable Anchor Kit

HSC Crossbar Control Anchor Kit

HSC Plug

Insurance L Code Justifications